Sources/Excerpts: Reviews, Cohort Studies and Randomised Controlled Trials Comparing the Feminising Efficacy of Estradiol Administered by Oral and Non-Oral Routes
By Sam | First published April 10, 2020 | Last modified October 5, 2020
Preface
This is a sources and excerpts supplement to the main article which can be found here.
Shah et al. (2014)
Shah, S., Forghani, N., Durham, E., & Neely, E. K. (2014). A randomized trial of transdermal and oral estrogen therapy in adolescent girls with hypogonadism. International Journal of Pediatric Endocrinology, 2014(1), 12. [DOI:10.1186/1687-9856-2014-12]:
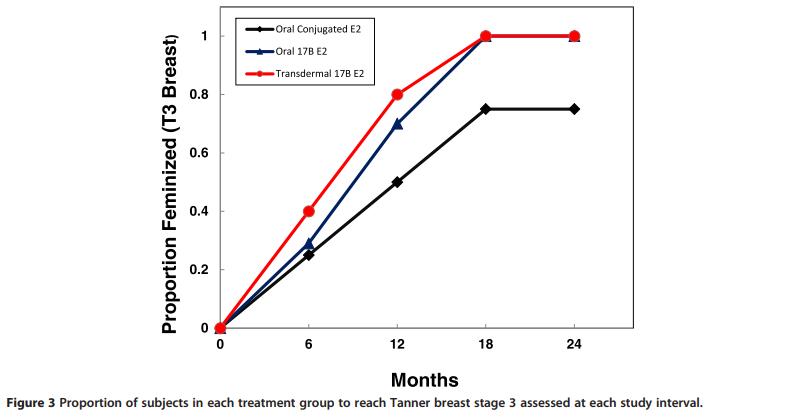
History and physical exams, including assessment of feminization by Tanner staging and breast diameter were obtained at baseline and every 6 months for 24 months.
The proportion of subjects in each group reaching Tanner 3 breast stage at each 6 month interval is shown in Figure 3. All subjects in the TBE and OBE group were feminized by 18 months, […]
Wierckx et al. (2014)
Wierckx, K., Van Caenegem, E., Schreiner, T., Haraldsen, I., Fisher, A. D., Toye, K., Kaufman, J. M., & T’Sjoen, G. (2014). Cross‐sex hormone therapy in trans persons is safe and effective at short‐time follow‐up: results from the European Network for the Investigation of Gender Incongruence. The Journal of Sexual Medicine, 11(8), 1999–2011. [DOI:10.1111/jsm.12571]:
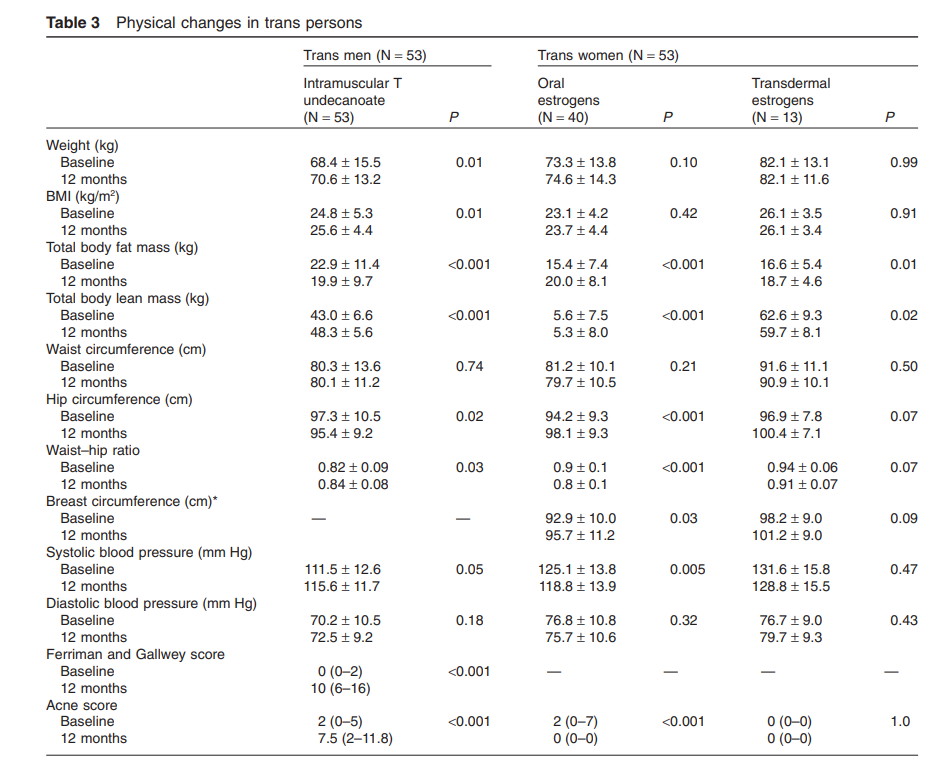
Antiandrogen with estrogen treatment resulted in a significant increase (average 3.3 cm) in breast circumference at the nipple, with a wide interindividual range of increase. Trans women using oral estrogens experienced similar changes in physical measures as those using transdermal estrogens (Table 3).
We found no changes in night sweats, abdominal pain, anxiety, irritability, palpitations, skin dryness, joint pain, muscle soreness, headache, mood swings, fatigue, concentration difficulties, memory, or sleep-related problems (data not shown). There were no significant differences in the presence of treatment related symptoms at the 3-month time point between trans women treated with oral or transdermal estrogen.
[…] In addition, trans women using oral EV showed significantly higher E1/E2 ratio than those using transdermal therapy. A lower E1/E2 ratio has been reported in postmenopausal women receiving transdermal hormone replacement therapy [40]. No other differences were observed between these two modes of estrogen treatment, suggesting both are equally effective.
de Blok et al. (2018)
de Blok, C. J. M., Klaver, M., Wiepjes, C. M., Nota, N. M., Heijboer, A. C., Fisher, A. D., Schreiner, T., T’Sjoen, G., & den Heijer, M. (2018). Breast development in transwomen after 1 year of cross-sex hormone therapy: results of a prospective multicenter study. The Journal of Clinical Endocrinology & Metabolism, 103(2), 532–538. [DOI:10.1210/jc.2017-01927]:
Because most transwomen (99%) in this study received cyproterone acetate as antiandrogen treatment, no analyses on treatment regimen other than oral vs transdermal estradiol administration route could be performed.
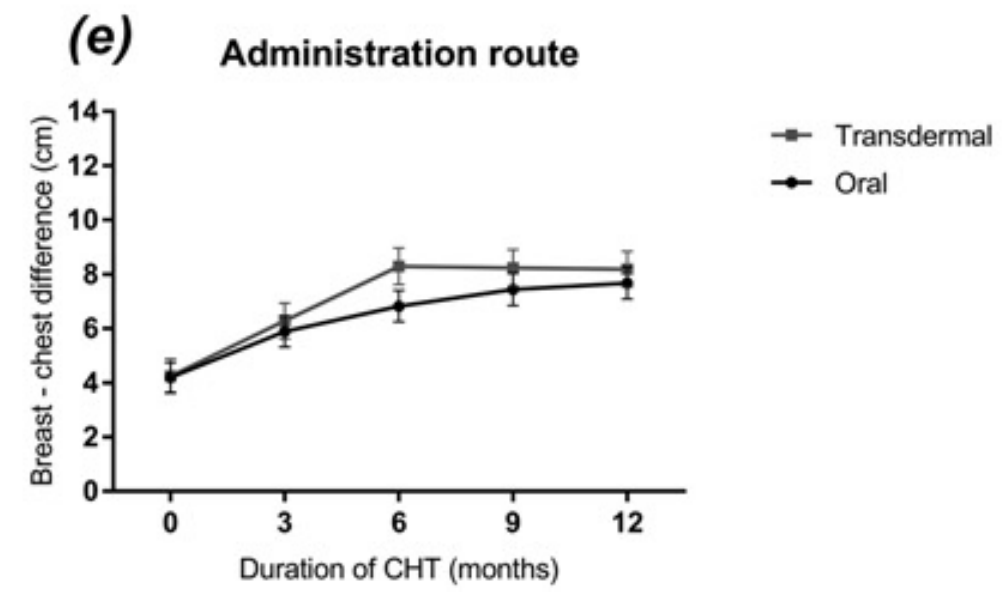
Transwomen treated with transdermal estradiol had a faster increase in breast-chest difference until 6 months after initializing treatment. However, breast development after 1 year of treatment did not differ between oral and transdermal estradiol administration [Fig. 5(e)].
Klaver et al. (2018)
Klaver, M., de Blok, C. J. M., Wiepjes, C. M., Nota, N. M., Dekker, M. J. H. J., de Mutsert, R., Schreiner, T., Fisher, A. D., T’Sjoen, G., & den Heijer, M. (2018). Changes in regional body fat, lean body mass and body shape in trans persons using cross-sex hormonal therapy: results from a multicenter prospective study. European Journal of Endocrinology, 178(2), 163–171. [DOI:10.1530/EJE-17-0496]:
Subgroups in transwomen were oral estradiol and transdermal estradiol. In these analyses, only transwomen and transmen using the same type of treatment during the whole year were included. Analyses on sex hormone levels were performed in quartiles of Z-scores, which is described in more detail below.
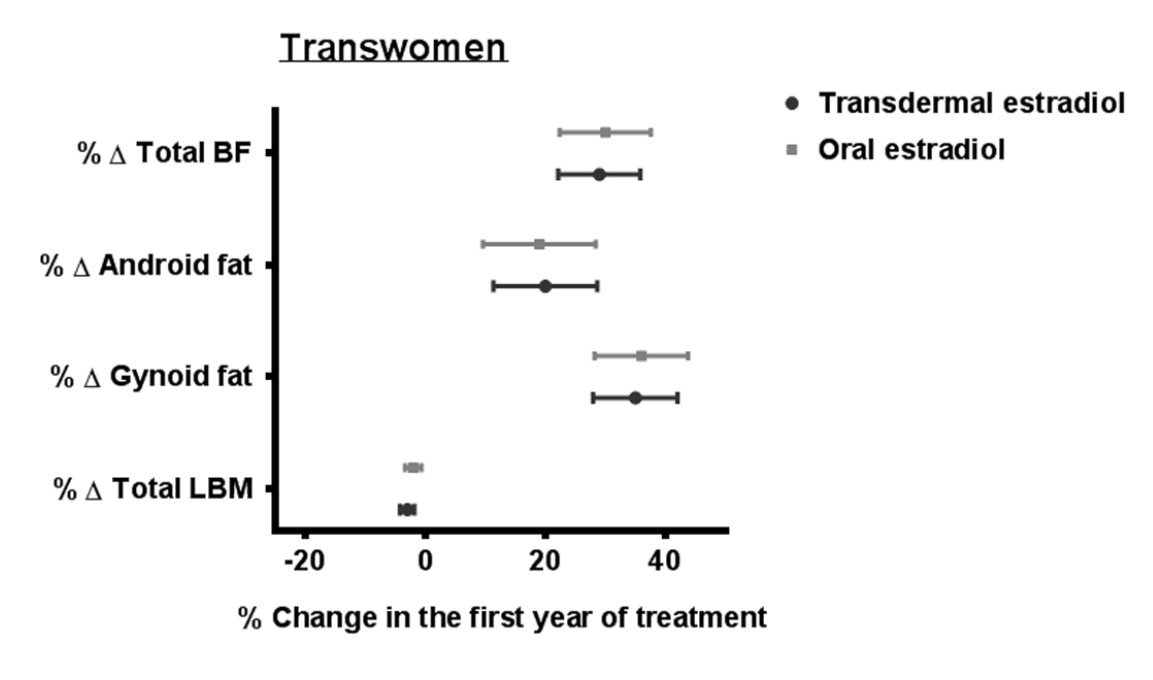
Sixty five transwomen using oral estrogens, 49 transwomen using transdermal estrogens, 41 transmen using T esters, 34 transmen using T gel and 30 transmen using T undecanoate were included. Crude analyses in transwomen showed a trend toward a larger change in some body fat outcomes in persons using oral estradiol compared with persons using transdermal estradiol, but after adjustment for BMI at the start and age, these differences disappeared (Supplementary Table 1).
Klein et al. (2018)
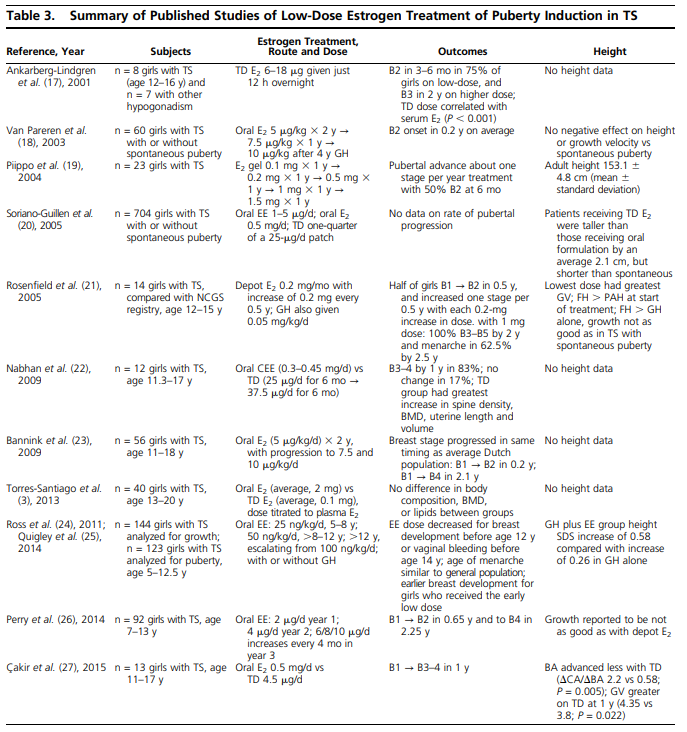
Klein, K. O., Rosenfield, R. L., Santen, R. J., Gawlik, A. M., Backeljauw, P. F., Gravholt, C. H., Sas, T. C. J., & Mauras, N. (2018). The Journal of Clinical Endocrinology & Metabolism, 103(5), 1790–1803. [DOI:10.1210/jc.2017-02183]:
Incremental dose increases at approximately 6-month intervals can mimic the normal pubertal tempo until adult dosing is reached over 2 to 3 years. This theoretically translates into a 25% to 100% increase in dose every 6 months for four to six dose changes between the initiation and adult doses portrayed in the Table 1. However, no studies to date have rigorously studied outcomes in relation to the rate of dose increase for the different preparations.
In general, the studies summarized in Table 3 report onset of breast buds within 6 months in most girls (17, 18, 22, 23, 27). Each of these regimens results in pubertal stage 4 breasts in an average of 2.25 years, which is similar to that in girls with TS who have spontaneous puberty (1.9 years), as well as in the general population (23).